Describe the Hybrid Orbitals Used by the Central Atom
However it has a much smaller. Get the detailed answer.

Solved Describe The Hybrid Orbitals Used By The Central Atom Chegg Com
Maximum of 2 electrons with opposite can be present in that overlapped space.
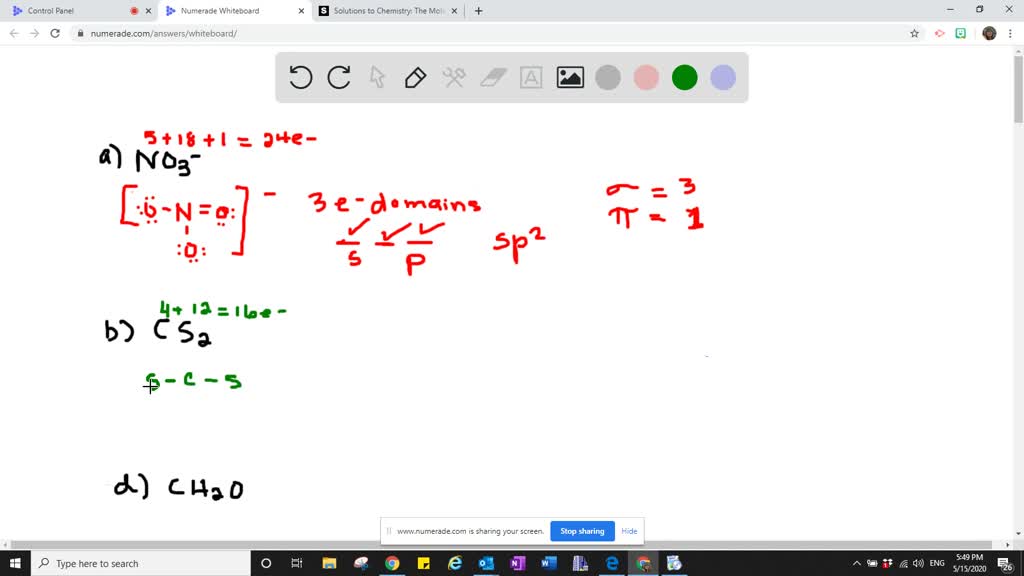
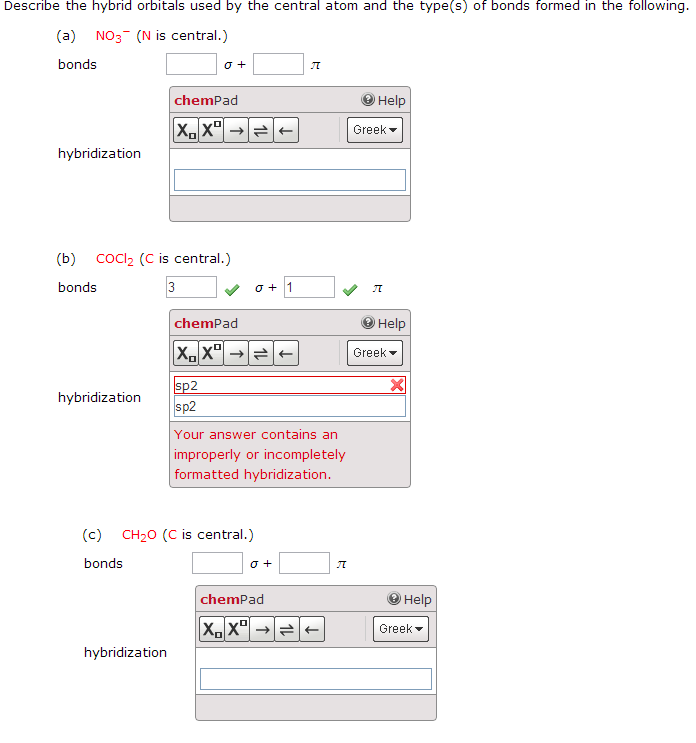
. ADescribe the hybrid orbitals used by the central atom in O3. Describe the hybrid orbitals used by the central atom and thetypes of bonds formed in each of the followinga NO3- N is the central atom ____σ ___π Hybridizationb CS2 C is the central atom ____σ ___π Hybridizationc CH2O C is the central atom ____σ ___π HybridizationPLEASE SHOW YOUR WORK. It is difficult to explain the shapes of even the simplest molecules with atomic orbitals.
ADescribe the hybrid orbitals used by the central atom in SO2. A covalent bond is formed when two atoms come close to each other their orbitals overlap and a pair of electrons come to that overlapped space. One sigma and two pi bonds Molecular orbitals are used to describe molecules like atomic orbitals describe quantum mechanical number can hold two electrons with opposite.
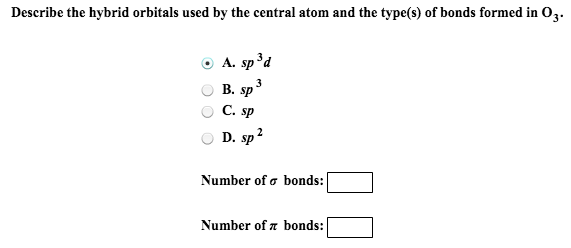
ADescribe the hybrid orbitals used by the central atom in O3. Orbitals used for bonding that are formed by mixing atomic orbitals from the same atom Hybrid orbitals are formed by the combination or mixing of -----orbitals from a specific atom. What is the number of sigma and pi bonds present in SO2.
Valence orbitals in atoms form new hybrid orbitals in molecule. 600 Posted By. In which of the following compounds will the molecules not form hydrogen bonds with each other.
Sailormaries7763 sailormaries7763 04282020 Chemistry High School answered Be sure to answer all parts. Get the answers you need now. 23 Check my work Be sure to answer all parts.
333 points Describe the hybrid orbitals used by the central atom s and the type. Hybrid Atomic Orbitals. 1124 Describe the hybrid orbitals used by the central atoms and the types of from INFORMATIC 123 at Indonesia University of Education.
Which types of atomic orbitals of the central atom mix to form hybrid orbita 0527 Use partial orbital diagrams to show how the atomic orbitals of the central. FatiMariannonalia fatiMariannonalia 12052016 Chemistry High School answered Describe the hybrid orbitals used by the central atom and the types of bonds formed in o3 1. Describe the hybrid orbitals used by the central atom and the types of bonds formed in I3.
EBook Number of bonds. Sp 3 d and sp 3 d 2 Hybridization. Which one of the following molecules is polar.
BWhat is the number of sigma and pi bonds present in O3. 333 points Describe the hybrid orbitals used by the central atom s and the type s of bonds formed in SO2. Two sp orbitals two p-orbitals unhybridized nitrogen is sp- hybridized triple bond.
Find step-by-step Chemistry solutions and your answer to the following textbook question. In the current case of carbon the single 2s orbital hybridizes with the three 2p orbitals to. A solution to this problem was proposed by Linus Pauling who argued that the valence orbitals on an atom could be combined to form hybrid atomic orbitals.
Course Title INFORMATIC 123. 11082015 0241 PM Due on. Up to 256 cash back Get the detailed answer.
1124 describe the hybrid orbitals used by the central. School Indonesia University of Education. Indicate the type of hybrid orbitals used by the central atom in CCl4.
23 Check my work Be sure to answer all parts. The number of hybrid orbitals formed is----- tothan the number of orbitals mixed and the ------ of hybrid orbital varies according to the specific orbitals mixed. Hybridization must be applied to the central atom to make equivalent bonds hybrid orbitals used.
Chemistry questions and answers. Describe the hybrid orbitals used by the central atom and the types of bonds formed in i3. Describe the hybrid orbitals used by the central atom and the types of bonds formed in a NO3-.
Describe the hybrid orbitals used by the central atom and the numbers of bonds sigma and pi formed in O3. Describe the hybrid orbitals used by the central atom and the types of bonds formed in i3. Describe the hybrid orbitals used by the central atom and the types of bonds formed in o3 Get the answers you need now.
Sulfur is in the same group as oxygen and H 2 S has a similar Lewis structure. In other words s and p orbitals can hybridize but p orbitals cannot hybridize with other p orbitals. 12082015 Question 00131031 Subject Chemistry Topic General Chemistry Tutorials.
Hybrid orbitals are the atomic orbitals obtained when two or more nonequivalent orbitals form the same atom combine in preparation for bond formation. Do long chains of hydrocarbons have strong IMF or weak IMF. 1045 which is more consistent with sp 3 hybrid orbitals 1095 on the central atom than with 2p orbitals 90.
Describe the hybrid orbitals used by the central atom and the types of bonds formed in i3. Some basics of the Valence Bond theory. To describe the five bonding orbitals in a trigonal bipyramidal arrangement.
You describe the molecular geometry around each central atom separately. Describe the hybrid orbitals used by the central atom and the types of bonds. Sp sp2 sp3 sp3d number of σ.
Solution for Describe the hybrid orbitals used by the central atoms andthe types of bonds formed in a BrF₃. GET 20 OFF GRADE YEARLY SUBSCRIPTION. LIMITED TIME OFFER.
Strong short gases medium liquids long solids. The geometry of a BeF 2 molecule can be explained for example by mixing the 2s orbital on the beryllium atom.

Solved Describe The Hybrid Orbitals Used By The Central Atom And The Type S Of Bonds Formed In A Mathrm No 3 B Mathrm Cs 2 Mathrm C Mathrm Ch 2 Mathrm O

Solved Be Sure To Answer All Parts Describe The Hybrid Chegg Com
Solved Describe The Hybrid Orbitals Used By The Central Atom Chegg Com
Solved Describe The Hybrid Orbitals Used By The Central Atom Chegg Com
No comments for "Describe the Hybrid Orbitals Used by the Central Atom"
Post a Comment